P NMR at the SMRL
Phosphorous-31 NMR spectroscopy (also known as P NMR, P-NMR, P-31 NMR, 31P NMR, 31P-NMR)
has substantially advanced the knowledge of phosphorus (P) compound content in soil, water
and other environmental samples. For the past ten years, the Stanford Magnetic Resonance Laboratory has worked closely with
Dr. Barbara Cade-Menun
to establish the instrumentation and resources necessary to analyze such samples. Cade-Menun is an internationally recognized
expert in soil organic P in general, and specifically in characterizing organic P in soil and environmental samples using 31P
nuclear magnetic resonance spectroscopy (31P-NMR).
31P-NMR is a powerful approach that has been applied to a wide variety of
studies including: phosphorous transformation; agricultural, forest, and natural
ecosystems; particle size separations and humic substances; manure, compost,
sewage sludge and fertilizers; and freshwater, estuary and marine sediments.
Because 31P is the only naturally occurring P isotope (100% natural abundance),
all P species within a sample can potentially be detected by NMR spectroscopy.
Thus, if the NMR experiments were carefully run, the area under each peak
should be proportional to the number of that particular type of P nucleus,
allowing NMR spectroscopy to quantitatively identify different P forms in
a sample. Compound classes that can be identified by P NMR include phosphonates, orthophosphate, orthophosphate
monoesters, orthophosphate diesters, pyrophosphate, and polyphosphate.
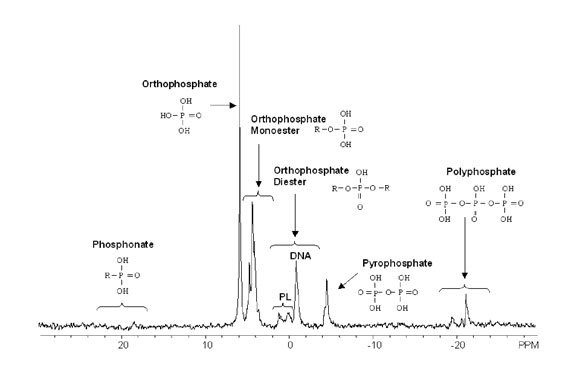
Figure used with permission from Cade-Menun, BJ (2005) Talanta, 66 359-371.
Examples of solution 31P-NMR spectra for several extracted environmental samples are shown below. These include: forest floor material from grass in an
oak-grass savannah in California; an alkaline agricultural soil from Idaho;
marine sediment trap material from Monterey Bay, California; a sewage
sludge sample from England; and a humic acid extracted from an Australian
soil. These give a good idea of the variation in P forms, and their relative
proportions, in environmental samples.
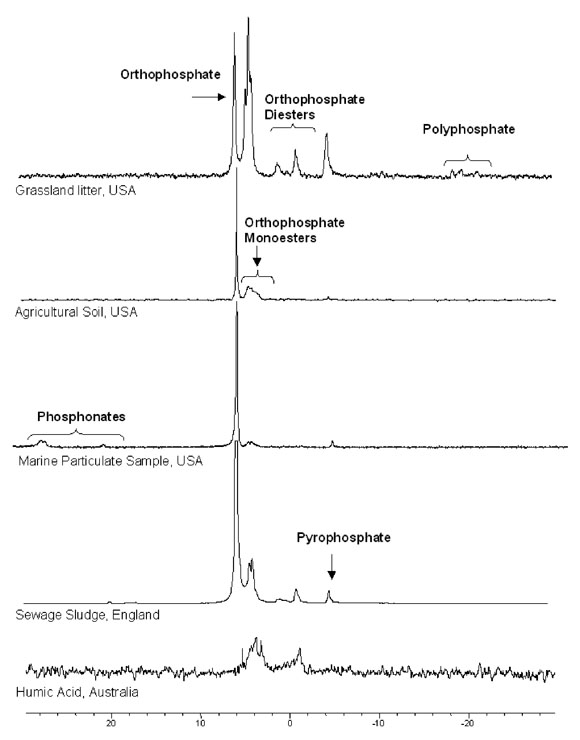
Figure used with permission from Cade-Menun, BJ (2005) Talanta, 66 359-371.
Instrumentation:
The SMRL 500 MHz Bruker Avance and 600 MHz Varian Inova are both equipped with 10 mm broadband detect probes tunable to 31P.
 
Protocol:
Typically provided to SMRL are:
- Samples as extracted and freeze dried (lyophilized) powders in 15 or 50 mL conical tubes. [See Cade-Menun et al. 2002 for extractant procedures]
- Extractant solution (typically 0.25 M NaOH, 0.05 M EDTA; 2-3 mL per sample).
- 10 N NaOH solution (2-3 mL per sample).
- D2O (NMR lock solvent; 1-2 mL per sample).
- 15 mL conicals (2 per sample).
- Any information available on total P content in samples.
- Any information on total Fe, Mn, content in samples.
Important sample considerations:
- Generally the higher concentration of phosphorous, the better. (Improved signal-to-noise, shorter experiments)
- However, sample viscosity can be an issue if trying to dissolve too much material. (Broader signals, poorer peak resolution)
- Samples high in paramagnetic species (Fe, Mn) also result in broader signals.
Typical sample treatment for NMR:
- Freeze dried powder is solvated with 1 mL D2O, 0.5-1 mL extractant solution (or H2O, depending on Fe, Mn content), 0.5 mL 10 N NaOH.
- Sample is repeatedly vortexed and allowed to sit over a 10 minute period to enhance solvation.
- If sample provided in 50 mL conical tube, sample is transferred to 15 mL conical tube, and 50 mL tube is rinsed with 0.5 mL extractant solution (or H2O) if necessary.
- Sample is centrifuged for 20 minutes at approximately 3300rpm/1380xG.
- Carefully transfer/decant liquid into 10 mm NMR tube. Pellet saved in tube in refrigerator as desired.
- Acquire NMR experiment.
- Transfer NMR sample to 15 mL conical tube and store in refrigerator.
 |
A 10 mm diameter NMR tube containing
3 mL of soil extract solution. |
Phytic acid (or other compound) spiking, if necessary/desired:
- Remove 0.25 mL (less if sample is dilute) of NMR sample and store in eppendorf tube in refrigerator.
- Spike NMR sample with equivalent amount of phytic acid spike solution (0.1 g phytic acid in 25 mL extractant solution).
- Reacquire NMR experiment.
Typical NMR experiment times per sample type (will vary depending on phosphorous content):
- Litter samples - 1 hour
- Soil samples - 4 hours
- Sediment samples - 8-12 hours
- Water samples - 12+ hours
Past and Present Collaborators:
AgResearch (New Zealand)
Invermay Agricultural Centre
Agriculture and Agri-Food Canada
Semiarid Prairie Agricultural Research Centre
Alabama A&M University
Department of Plant and Soil Science
Bournemouth University (U.K.)
The Centre for Ecology and Conservation Biology, School of Conservation Science
CSIRO Plant Industry (Australia)
Lincoln University (New Zealand)
Agriculture and Life Sciences
Centre for Soil and Environmental Quality
Old Dominion University
Department of Chemistry & Biochemistry
The Scottish Crop Research Institute(Scotland)
Smithsonian Tropical Research Institute (Panama)
South Florida Water Management District
Stanford University
Carnegie Institute
Department of Geological and Environmental Science
United States Department of Agriculture - Agricultural Research Service
New England Plant, Soil, and Water Laboratory (Maine)
Northwest Irrigation and Soils Research Lab (Idaho)
Pasture Systems and Watershed Management Research Unit (Pennsylvania)
University of Arkansas
Biological and Agricultural Engineering
University of California - Berkeley
College of Chemistry
Department of Environmental Sciences
University of Delaware
Department of Plant and soil Sciences
University of Idaho
Department of Plant, Soil and Entomological Sciences
University of Maine
Department of Plant Soil and Environmental Sciences
University of Otago (New Zealand)
Department of Chemistry
University of Pennsylvania
Section of Animal Production Systems, School of Vetinary Medicine
University of South Carolina
Department of Chemistry and Biochemistry
Department of Geological Sciences and Marine Science Program
University of Vermont
Department of Geology
Department of Plant and Soil Science
Environmental Microbiology and Biotechnology, School of Engineering
University of Western Australia(Australia)
University of Centre for Land Rehabilitation, School of Earth and Geographical Sciences
West Virginia University
Department of Biology
Yale University
Chemical Engineering, Environmental Engineering Program
Publications:
Methods and Review Papers:
Cade-Menun, B.J.; Liu, C.W.; Nunlist, R.; McColl, J.G. (2002)
"Soil and litter phosphorus-31 nuclear magnetic resonance spectroscopy: extractants, metals, and phosphorus relaxation times"
J. Environ. Qual., 31, 457-465.
Turner, B.L., B.J. Cade-Menun, L.M. Condron and S. Newman. (2005)
"Extraction of organic phosphorus from soil and manure."
Talanta, 66, 294-306.
Cade-Menun, BJ (2005)
"Characterization phosphorus in environmental and agricultural samples by 31P nuclear magnetic resonance spectroscopy"
Talanta, 66 359-371.
Studies:
He, Z., C.W. Honeycutt, T. S.Griffin, B.J. Cade-Menun, P.J. Pellechia, and Z. Dou. (2009)
"Phosphorus forms in organic and conventional dairy manure identified by solution and solid state P-31 NMR spectroscopy"
Journal of Environmental Quality, 38, in press.
He, Z., J. Mao, C.W. Honeycutt, T. Ohno, J.F. Hunt and B.J. Cade-Menun. (2009)
"Characterization of plant-derived dissolved organic matter by multiple spectroscopic techniques."
Biology and Fertility of Soils 45, 609-616.
Hill, J.E., and B.J. Cade-Menun. (2009)
"Phosphorus-31 nuclear magnetic resonance transect study of poultry operations on the Delmarva Peninsula."
Journal of Environmental Quality, 38, 130-138.
He, Z., C. W. Honeycutt, B. J. Cade-Menun, Z. N. Senwo, and I. A. Tazisong. 2008. Phosphorus in poultry litter and soil: enzymatic and nuclear magnetic resonance characterization Soil Science Society of America Journal 72:1425-1433.
McDowell, R.W., Dou, Z., Toth, J.D., Cade-Menun, B.J., Kleinman, P.J.A., Soder, K., and L. Saporito. 2008. A comparison of phosphorus speciation and potential bioavailability in feed and feces of different dairy herds using 31P nuclear magnetic resonance spectroscopy. Journal of Environmental Quality 37:741-752.
McDowell, R.W., B. Cade-Menun, and I. Stewart. 2007. Organic P speciation and pedogenesis: analysis by 31P nuclear magnetic resonance spectroscopy. European Journal of Soil Science 58:1348-1357.
He, Z., B.J. Cade-Menun, G.S. Toor, A. Fortuna, C.W. Honeycutt, and J.T Sims. 2007. Comparison of phosphorus forms in wet and dried animal manures by solution phosphorus-31 nuclear magnetic resonance spectroscopy and enzymatic hydrolysis. Journal of Environmental Quality 36:1086-1095.
Cade-Menun, B.J., J.A. Navaratnam and M.R. Walbridge. 2006. Characterizing dissolved and particulate phosphorus in water with 31P-NMR spectroscopy. Environmental Science and Technology 40:7874-7880.
McLaughlin, K., B.J. Cade-Menun and A. Paytan. 2006. The oxygen isotopic composition of phosphate in Elkhorn Slough, California: A tracer for phosphate sources. Estuarine, Coastal and Shelf Science 70: 499-506.
He, Z., T. Ohno, B.J. Cade-Menun, M.S. Erich, and C.W. Honeycutt. 2006. Spectral and chemical characterization of phosphates associated with humic substances. Soil Science Society of America Journal 70:1741-1751.
He, Z., T. Ohno, B. J. Cade-Menun, M. S. Erich, and C.W. Honeycutt. 2006. Forms and bioavailability of phosphorus associated with natural organic matter. Chinese Journal of Geochemistry 25 (Suppl. 1):259.
He, Z., C. W. Honeycutt, T. Ohno, J.F. Hunt, and B. J. Cade-Menun 2006. Phosphorus features in FT-IR spectra of natural organic matter. Chinese Journal of Geochemistry 25 (Suppl. 1): 259-260.
George, T.S., B.L. Turner, P.J. Gregory, B.J. Cade-Menun and A.E. Richardson. 2006. Depletion of organic phosphorus from oxisols in relation to phosphatase activities in the rhizosphere. European Journal of Soil Science 57:47-57.
McDowell, R.W., I. Stewart and B.J. Cade-Menun. 2006. An examination of spin-lattice relaxation times for analysis of soil and manure extracts by liquid state phosphorus-31 nuclear magnetic resonance spectroscopy. J Journal of Environmental Quality 35: 293-302.
Smith, M. T. E., B. J. Cade-Menun and M. Tibbett. 2006. Soil phosphorus dynamics from sewage sludge at different stages in a treatment stream. Biology and Fertility of Soils 42: 186-197.
Cade-Menun, B.J., A. Paytan, C.R. Benitez-Nelson and P. Pellechia. 2005. Refining 31P nuclear magnetic resonance spectroscopy for marine particulate samples: storage conditions and extraction recovery. Marine Chemistry. 97:293-306.
Toor, G.S., B.J. Cade-Menun and J.T. Sims. 2005. Establishing a linkage between phosphorus forms in dairy diets, feces and manures. Journal of Environmental Quality 34:1380-1391.
Jeremy C. Hansen, Barbara J. Cade-Menun, and Daniel G. Strawn (2004)
"Phosphorus Speciation in Manure-Amended Alkaline Soils"
J. Environmental Quality, 33 1521-1527.
Toor, G.S., L.M. Condron, B.J. Cade-Menun, H.J. Di, and K.C. Cameron. 2005. Preferential phosphorus leaching from an irrigated grassland soil. European Journal of Soil Science.56: 155-168.
Toor, GS; Condron, LM; Di, HJ; Cameron, KC; Cade-Menun, BJ (2003)
"Characterization of organic phosphorus in leachate from a grassland soil"
SOIL BIOLOGY & BIOCHEMISTRY, 35 1319-1325.
Turner, B.L., B. J. Cade-Menun and D.T. Westermann. 2003. Organic phosphorus composition and potential bioavailability in calcareous soils of the western United States. Soil Science Society of America Journal 67: 1168-1179.
Adina Paytan, Barbara J. Cade-Menun, Karen McLaughlin and Kristina L. Faul. 2003. Selective phosphorus regeneration of sinking marine particles: evidence from 31P-NMR. Virtual Journal of Geobiology, Volume 2, Issue 6, on the web at http://earth.elsevier.com/geobiology/
Paytan, A; Cade-Menun, BJ; McLaughlin, K; Faul, KL (2003)
"Selective phosphorus regeneration of sinking marine particles: evidence from P-31-NMR"
MARINE CHEMISTRY, 82 55-70.
Book Chapters / Other:
O'Halloran, I.P., and B.J. Cade-Menun. 2008. Chapter 24. Total and organic phosphorus. pp. 265-291.In: Carter, M.R., and E.G. Gregorich, eds. Soil Sampling and Methods of Analysis, 2nd Edition. Canadian Society of Soil Science and CRC Press.
Condron, L.M., B.L. Turner and B.J. Cade-Menun. 2005. Chapter 4. Chemistry and dynamics of soil organic phosphorus. pp. 87-121. In: J.T. Sims and A.N. Sharpley, eds. Phosphorus, Agriculture and the Environment. Monograph no 46. Soil Science Society of America. Madison, WI.
Cade-Menun, B.J. 2005. Using phosphorus-31 nuclear magnetic resonance spectroscopy to characterize phosphorus in environmental samples. In: B.L. Turner, E. Frossard and D. Baldwin, eds. Organic Phosphorus in the Environment. CABI Publishing. pp 21-44.
|




