| HOME | INSTRUMENTATION | NMR TIME | USERS & RESEARCH |
INDUSTRIAL AFFILIATES |
NEWS & LINKS |
Publications: (Details Below)
Li, Q; Khosla, C; Puglisi, JD; Liu, CW (2003) "Solution structure and backbone
dynamics of the holo form of the frenolicin acyl carrier protein" BIOCHEMISTRY,
42 4648-4657.
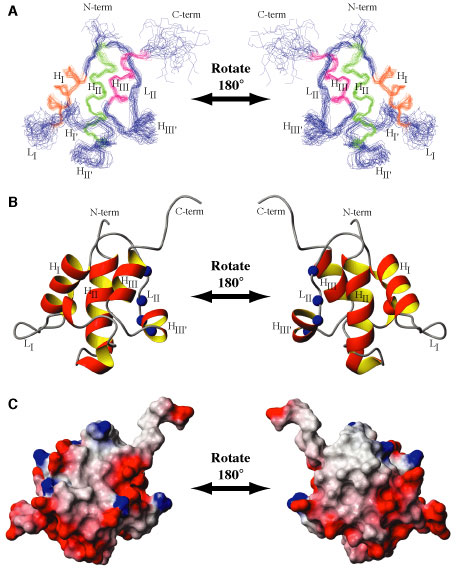
Details: Solution structure and backbone dynamics of the holo form of the frenolicin acyl carrier protein Li, Q.; Khosla, C; Puglisi, JD; Liu, CW (2003) BIOCHEMISTRY, 42 4648-4657. Abstract: During polyketide biosynthesis, acyl carrier proteins (ACPs) perform the central role of transferring polyketide intermediates between active sites of polyketide synthase. The 4'-phosphopantetheine prosthetic group of a holo-ACP is a long and flexible arm that can reach into different active sites and provide a terminal sulfhydryl group for the attachment of acyl groups through a thioester linkage. We have determined the solution structure and characterized backbone dynamics of the holo form of the frenolicin acyl carrier protein (fren holo-ACP) by nuclear magnetic resonance (NMR). Unambiguous assignments were made for 433 hydrogen atoms, 333 carbon atoms, and 84 nitrogen atoms, representing a total of 94.6% of the assignable atoms in this protein. From 879 meaningful NOEs and 45 angle constraints, a family of 24 structures has been calculated. The solution structure is composed of three major alpha-helices packed in a bundle with three additional short helices in intervening loops; one of the short helices slowly exchanges between two conformations. Superposition of the major helical regions on the mean structure yields average atomic rmsd values of 0.49 +/- 0.09 and 0.91 +/- 0.08 Angstroms for backbone and non-hydrogen atoms, respectively. Although the three-helix bundle fold is conserved among acyl carrier proteins involved in fatty acid synthases and polyketide synthases, a detailed comparison revealed that ACPs from polyketide biosynthetic pathways are more related to each other in tertiary fold than to their homologues from fatty acid biosynthetic pathways. Comparison of the free form of ACPs (NMR structures of fren ACP and the Bacillus subtilis ACP) with the substrate-bound form of ACP (crystal structure of butyryl-ACP from Escherichia coli) suggests that conformational exchange plays a role in substrate binding.
|
| Bio-X | Structural Biology | School of Medicine | Stanford University |