| HOME | INSTRUMENTATION | NMR TIME | USERS & RESEARCH |
INDUSTRIAL AFFILIATES |
NEWS & LINKS |
Publications:
Millhauser, GL; McNulty, JC; Jackson, PJ; Thompson, DA; Barsh, GS; Gantz, I (2003)
"Loops and links: Structural insights into the remarkable function of the agouti-related protein"
MELANOCORTIN SYSTEM, 994 27-35.
Jackson, PJ; McNulty, JC; Yang, YK; Thompson, DA; Chai, BX; Gantz, I; Barsh, GS; Millhauser, GL (2002)
"Design, pharmacology, and NMR structure of a minimized cystine knot with agouti-related protein activity"
BIOCHEMISTRY, 41 7565-7572.
(Details Below)
McNulty, J.C., Thompson, D.A., Bolin, K.A., Wilken, J., Barsh, G.S., and Millhauser,
G.L. (2001) "High-Resolution NMR Structure of the Chemically-Synthesized Melanocortin
Receptor Binding Domain AGRP(87-132) of the Agouti-Related Protein"
Biochemistry, 40 15520-15527.
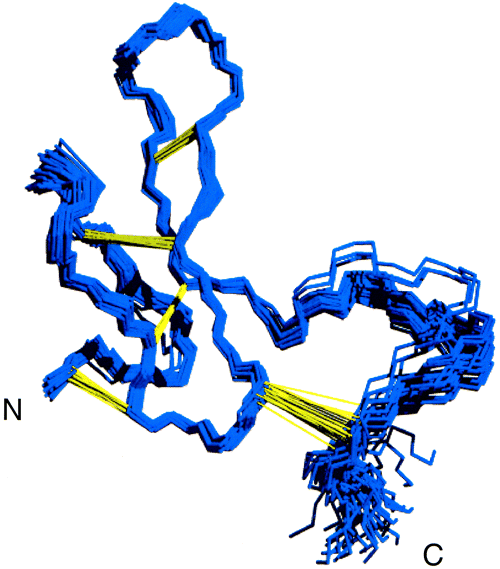
Details: High-Resolution NMR Structure of the Chemically-Synthesized Melanocortin Receptor Binding Domain AGRP(87-132) of the Agouti-Related Protein McNulty, J.C., Thompson, D.A., Bolin, K.A., Wilken, J., Barsh, G.S., and Millhauser, G.L. (2001) Biochemistry ASAP Article. Abstract: The agouti-related protein (AGRP) is an endogenous antagonist of the melanocortin receptors MC3R and MC4R found in the hypothalamus and exhibits potent orexigenic (appetite-stimulating) activity. The cysteine-rich C-terminal domain of this protein, corresponding to AGRP(87-132), contains five disulfide bonds and exhibits receptor binding affinity and antagonism equivalent to that of the full-length protein. The three-dimensional structure of this domain has been determined by 1H NMR at 800 MHz. The first 34 residues of AGRP(87-132) are well-ordered and contain a three-stranded antiparallel
|
| Bio-X | Structural Biology | School of Medicine | Stanford University |